Center for Integrated Nanostructure Physics, Research Team of Professor Hyo-Young Lee, Developed a Hydrogen Production Catalyst that 20 Times Cheaper but Lasts Longer
- Promote water electrolysis with low-cost metal… Expect lower hydrogen production costs -
The research team led by Professor Hyo-Young has developed a water decomposition catalyst that is about 6 times more productive and lasts at least 4 times longer with 20 times cheaper costs. It is expected that this will contribute to the supply of eco-friendly hydrogen by drastically reducing the cost of electrolysis of water.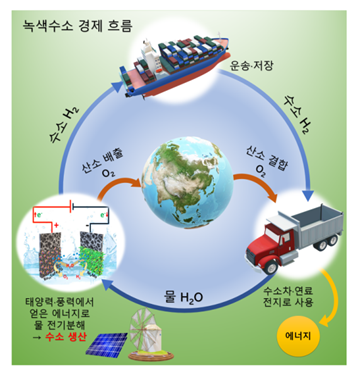
[Image 1] Green Hydrogen Economy
Hydrogen obtained through electrolysis is a clean source of energy that does not emit carbon, and can serve as an efficient energy carrier that carries energy cheaply.
The research team developed a catalyst by attaching oxygen atoms on cobalt, iron, and a tiny amount of ruthenium (Ru), which are inexpensive metastatic metals, and has excellent performance and can last at least 100 hours.
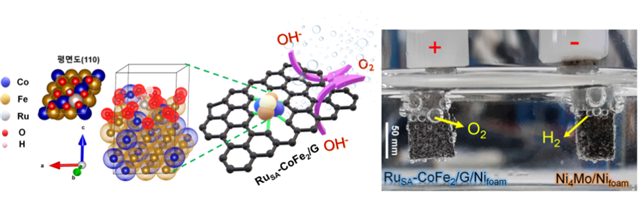
[Image 2] Developed electro-catalyst crystal structure and water decomposition process
(left) developed crystalline structure of catalytic alloys. Attached oxygen (red) to the surface. (right) produce oxygen and hydrogen through an electric water decomposition reaction.
They predicted that pre-absorption of oxygen on the catalytic surface would stabilize OOH*. Accordingly, the experiment was conducted by making cobalt-iron alloys on the surface. The result confirmed that the highest oxygen generation was when eight oxygen atoms were attached to the catalytic crystal. In addition, the Ruthenium atom was added to reduce the energy barrier in the speed determination stage and attached it to a porous carbon layer with high electrical conductivity.
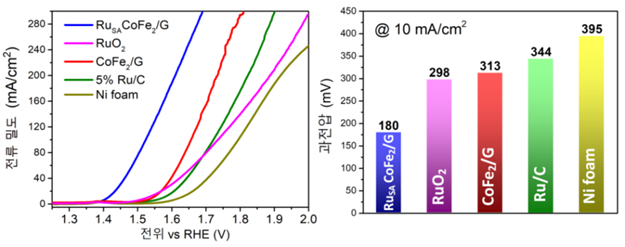
[Image 3] Evaluation of developed high efficiency electric catalyst performance
(left) The newly developed catalyst (blue) shows six times higher current density, or oxygen, at the same potential. Because of this, the performance of oxygen generation is far superior to that of conventional catalysts.
(right) The catalyst in this study (blue) reaches the same current density (small oxygen) at the lowest voltage compared to the previous catalyst, which requires the least energy.
The developed catalyst had about six times more production than the previous one, and was able to generate oxygen at a much lower voltage. The faster the rate of oxygen generation, the greater the current density, and the existing Ruthenium oxide (RuO2) required 298 mV to obtain a current density of 10 mA/cm2. On the other hand, the electric catalyst developed by the researchers requires 180 mV. This means that it is energy efficient because it can decompose water at low voltage.
Additionally, this catalyst could be maintained at least 100 hours. Existing Ruthenium oxide catalysts were oxidized well, making it difficult to maintain performance for more than 24 hours. The cobalt-iron alloy used this time was less oxidized, so there was no structural change even after 100 hours.
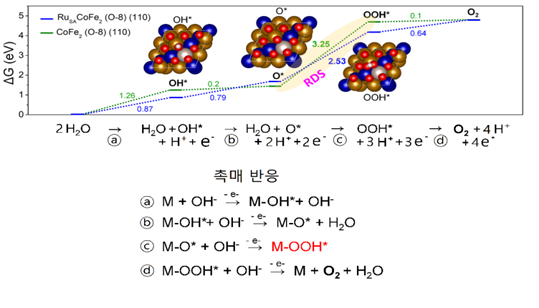
[Image 4] Oxygen-generated reaction mechanism
Professor Hyo-Young said that “The task of making eco-friendly hydrogen cheaper than by-product hydrogen of petroleum and coal through water decomposition has long been limited. By developing low-cost, high-efficiency, oxygen-producing catalysts, we expect to take a step closer to the environment-friendly hydrogen economy of decarbonization.”
The research outcomes were published online on November 4 in a world-renowned journal, Energy & Environmental Science (IF 30.287).
[Related articles]
- https://biz.chosun.com/site/data/html_dir/2020/11/24/2020112400964.html?utm_source=naver&utm_medium=original&utm_campaign=biz
- https://newsis.com/view/?id=NISX20201124_0001244668&cID=13001&pID=13000